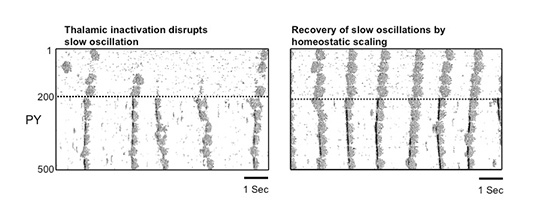
During natural slow-wave sleep (SWS), brain activity recorded from electroencephalogram (EEG) is characterized by large-amplitude fluctuations of field potential, which reflect synchronous alternating periods of activity (cortical Up states) and silence (cortical Down states) across the thalamocortical system. Recovery of slow oscillations after extensive thalamic lesions and the absence of slow oscillations in the thalamus of decorticated cats pointed to an intracortical origin for this rhythm. If was suggested that thalamic neurons play a merely secondary role simply following intrinsic cortical activity. Recent in vivo studies revealed, however, that following QX-314 injection to the dorsal thalamus, the corresponding cortical neurons became quiet except for occasional and rare active states. In this study, using large-scale models of the thalamocortical system, the role of thalamic input during slow sleep oscillation was systematically investigated. With intact thalamus the entire network displayed rhythmic slow (<1 Hz) oscillation driven by occasional summation of action-potential independent excitatory synaptic potentials (minis) in cortical neurons; these oscillations were synchronized despite variations of the minis rate and amplitude across network sites. When the thalamocortical projections were blocked, cortical neurons were found to become mostly quiet at particular network sites where the spontaneous miniature synaptic activity was low. Only occasional active states were generated at low rate at these sites while the rest of the network displayed normal slow oscillation. Increasing the strength of excitatory intracortical connections to model effects of homeostatic plasticity recovered slow oscillation in the entire network. This study suggests that thalamocortical input plays a significant role in maintaining and synchronization of slow oscillation and may directly contribute to various brain functions developed during the slow-wave sleep.
Share
APR
