Mechanisms and functional role of sleep in memory and learning
During slow-wave sleep, the cortex is decoupled from sensory inputs, and can be devoted to consolidating previously acquired labile memories into stable memories. The goal of our research is to combine animal, human and computational works to explain mechanisms of sleep rhythm generation and how sleep rhythms contribute to memory consolidation.
Projects:
Can sleep protect memories from catastrophic forgetting?
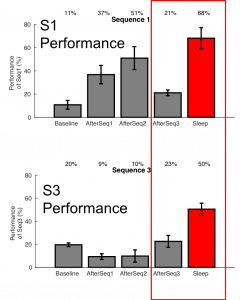 Continual learning remains to be an unsolved problem in artificial neural networks. Biological systems have evolved a mechanism by which they can prevent catastrophic forgetting of old knowledge during new training and allow lifelong learning. Building upon data suggesting the importance of sleep in learning and memory, we test a hypothesis that sleep plays a role in protecting memories from catastrophic forgetting. We found that training in a thalamocortical network of a “new” memory that overlaps with previously stored “old” memory results in degradation of the old memory. Simulating sleep state immediately after new learning led to replay of memories, which protected the old memory from forgetting and ultimately enhanced both memories. We show that slow-wave sleep results in the fine tuning of synaptic connectivity to allow for the same population of neurons to store competing memories without the need of explicit retraining of previously stored memory sequences. Our results predict that oscillatory activity in the thalamocortical network during sleep reorganizes synaptic connectivity to maximize separation between groups of synapses responsible for conflicting memories that reduces interference and enables brain networks to undergo continual learning.
Continual learning remains to be an unsolved problem in artificial neural networks. Biological systems have evolved a mechanism by which they can prevent catastrophic forgetting of old knowledge during new training and allow lifelong learning. Building upon data suggesting the importance of sleep in learning and memory, we test a hypothesis that sleep plays a role in protecting memories from catastrophic forgetting. We found that training in a thalamocortical network of a “new” memory that overlaps with previously stored “old” memory results in degradation of the old memory. Simulating sleep state immediately after new learning led to replay of memories, which protected the old memory from forgetting and ultimately enhanced both memories. We show that slow-wave sleep results in the fine tuning of synaptic connectivity to allow for the same population of neurons to store competing memories without the need of explicit retraining of previously stored memory sequences. Our results predict that oscillatory activity in the thalamocortical network during sleep reorganizes synaptic connectivity to maximize separation between groups of synapses responsible for conflicting memories that reduces interference and enables brain networks to undergo continual learning.
Oscillations in large-scale neural networks
We develop a new computationally efficient approach to analyze large-scale networks of biological neurons. This approach is based on using difference equations (map) for simulation of neuron dynamics. The nonlinear maps produce very rich spectrum of dynamical behaviors while remaining simple and low-dimensional systems and, therefore, can be very computationally efficient. Conventional approach based on simulating ordinary differential equations (such as Hodgkin-Huxley type models) quickly reaches its limit when the number of elements in the network increases. It makes this approach impractical for studying those problems when the analyzed phenomena originate from a collective behavior of large neural ensembles. A map-based model of a neuron that realistically replicates the dynamical mechanisms underlying both its spiking and bursting activity and correctly captures the input-output processes opens new opportunities in the studies of large-scale network functionality. This approach will provide the basis for network simulations of different brain systems, including hundreds of thousands neurons, at the realistic time scales using conventional workstations.
Example of C++ code to simulate spiral wave dynamics in 2D network of regular spiking neurons and fast spiking interneurons:
C++ code to simulate 2D network – network2D.cpp.txt
Input file for network simulations – input2D.txt
To compile the code using GCC compiler: gcc network2D.cpp -lm -O2 -o network2D
To run simulations: network2D input2D.txt > tmp
Matlab code to simulate a network of 90 map-based RS neurons connected along a chain using excitatory connections is available here. It replicates fig 6 in N.Rulkov, I.Timofeev and M.Bazhenov. Oscillations in large-scale cortical networks: map-based model. Journal of Computational Neuroscience 17, 203�223, 2004
Main code: NetworkSim2.m
Function to calculate synaptic currents: Isynaptic.m
Function to simulate a regular spiking neuron (RS): RS2.m
Function to simulate a Fast spiking neuron (FS): FS2.m
LabView applet (MS Windows version) to simulate in real time response to DC pulse of a RS type map-based neuron is available here. Please install and run as Windows application: 2D-Map-RS
Click here to see movie of spiral wave dynamics. These neuron and network models are discussed inN.Rulkov, I.Timofeev and M.Bazhenov. Oscillations in large-scale cortical networks: map-based model. Journal of Computational Neuroscience 17, 203�223, 2004
Mechanisms of ripple generation and sequence replay in the hippocampus
Among hippocampal-specific activity patterns, sharp-wave ripple complexes are brief high-frequency events, during which the firing sequences of previously activated cells are re-played. It is believed that sequence reactivation during ripples contributes to memory formation in awake patterns and to memory consolidation during sleep. Ripples in the pyramidal layer of hippocampal area CA1 are thought to be triggered by a generalized excitatory event in area CA3.
We develop computational models of ripple generation to explore hippocampal sequence replay during sleep. In our model, perisomatic interneurons are organized in brief oscillatory transients by common excitation, and such high-frequency firing mediates high-frequency LFP oscillations in pyramidal neurons. Pyramidal cells are then firing within the windows of opportunity left by the inhibitory activity. We hypothesize an essential role for axo-axonic cell activity in regulating sequence replay.
Neuronal Plasticity during Slow Wave Sleep Oscillations
Sleep is critical for regulation of synaptic efficacy, consolidation of memories and learning. We develop thalamocortical network models of the slow-wave sleep activity characterized by repeatable (< 1 Hz) transitions between active (Up) and silent (Down) states of the network. Spike-timing dependent synaptic plasticity is implemented to regulate synaptic efficacy, which is associated with the sequence replay. We study how interaction between cortically generated slow waves and sparse external input, possibly representing input from hippocampal formation, may lead to reorganization of synaptic strength during stage 3/4 sleep.
Effect of Sleep Spindle Oscillations on Memory Consolidation
Sleep facilitates the consolidation of memories. The number of sleep spindles (transient neural events in non rapid eye movement (NREM) sleep, 9–15 Hz) in a post-training sleep period correlates with the magnitude of declarative memory improvement (e.g., conscious, episodic memories), whereas minutes in REM sleep correlate with improvement in non-declarative memories (e.g., unconscious, perceptual or sensorimotor skills).
Although the studies report that individual sleep features correlate with improvement in specific memory domains, we do not know to what extend manipulating these sleep features will lead to changes in these precise memory domains. Using human EEG data, pharmacological interventions and sophisticated signal processing we investigate the specificity of sleep-dependent memory.
