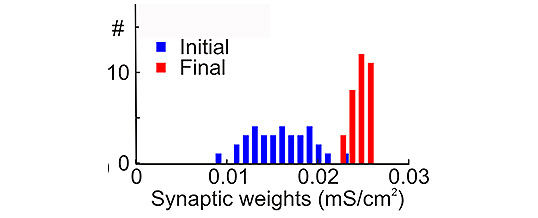
Spike timing dependent plasticity (STDP) modifies synaptic strength depending on the relative timing of pre-synaptic input and post-synaptic spikes. The effects of STDP in computational models often lead to physiologically inaccurate or unstable distributions of synaptic strength. Examples of stable but physiologically unrealistic synaptic strength distributions include bimodal distributions and highly skewed distributions. Connection strengths also tend to be excessively unstable for learning. Preventing this “run-away” dynamics using basic STDP mechanisms requires fine tuning of learning rules and network parameters that define firing patterns of model neurons. These results suggested the necessity for additional plasticity mechanisms. One candidate form of plasticity that can resolve these issues is heterosynaptic plasticity – i.e. changes at synapses which were not active during plasticity induction. Here we used a combination of in vitro experiments to study rules of heterosynaptic plasticity at excitatory neocortical synapses and computer modeling to explore if heterosynaptic plasticity, implemented according to the rules found in experiments, can override “run-away” effects of STDP and stabilize synaptic weights distribution. In vitro intracellular tetanization – experimental model of heterosynaptic plasticity – led to the changes of synaptic strength in weight specific manner. Synapses with initially low release probability had a tendency to be potentiated, while synapses with initially high release probability had tendency to be depressed or did not change. These experimental rules have been implemented in conductance-based model of a cortical neuron receiving inputs via excitatory AMPA-type synapses. Presynaptic activity was simulated using Poisson distributed spike trains with different degree of correlation between inputs. STDP implemented in the model included dependence on intracellular calcium concentration and on the current synaptic strength. These synaptic rules enabled model synapses to maintain unimodal distribution of synaptic weights. Results of our study suggest that heterosynaptic plasticity may control synaptic dynamics and play critical role in achieving stable yet adaptable memory storage.
Share
OCT
