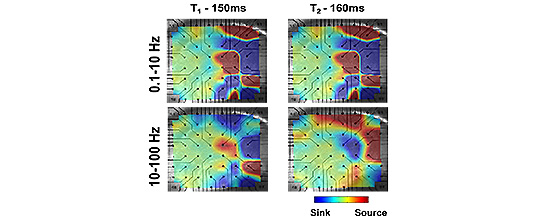
The role of changes in ion concentrations in epilepsy remains unclear. It had been long known that increase of [K+]o (Hodgkin and Horovicz, 1959) and/or decrease of [Ca2+]o (Frankenhauser and Hodgkin, 1957) lead to neuronal hyperexcitability and possible epilepsy. But, in epilepsy, the exact mechanisms of the ionic changes, and the contributions of network dynamics remain to be clarified. Here we used multi electrode array (MEA) recordings combined with K-selective electrode and optical measurements from in vitro hippocampal mouse slices to gain insight into the spatio-temporal distribution of epileptiform activity in different frequency domains. We used elevated [K+]o (10mM), decreased [Ca2+]o (0 mM unbuffered) and specific GABAa-receptors inhibitor Bicuculine (100uM) to induce seizure-like activity, which consisted of a series of spontaneous hyperactivity episodes that repeated every few minutes. Each episode lasted ~30 sec and included multiple network events with a characteristic frequency of .2-1 Hz. In turn, each event consisted of a few intraevent spikes (10-100 Hz) and high frequency oscillations (100-1000 Hz). We hypothesize that the low range of the frequency spectrum likely reflects slow synaptic currents synchronized during epileptiform generation, since it is completely abolished by bath application of synaptic inhibitor (5uM NBQX). Oscillations in the mid frequency range arise from local network synchronization due to the inhibitory feedback mediated by GABAergic interneurons. The high frequency oscillation likely represents multiunit activity. Cross-coherence analysis revealed reductions in coherence for high frequencies, while the coherence in low and mid frequencies increased during the epileptiform events. Propagation of the activity across the hippocampus was confirmed by MEA analysis, which revealed systematic delays in the initiation of epileptiform events recorded between 60 electrodes. We conclude that a decrease in reliability of synaptic transmission reduces long-range synchrony of spiking of individual cells during epileptiform activity. On the other hand, an increase in low-frequency band coherence between channels suggests synchronized development of the low-frequency activity characteristic of the spatio-temporal patterns of seizure.
ShareMAY
